FDA is Recommending Pausing the Use of Johnson and Johnson Vaccine
CDC & FDA Recommend Pausing Johnson & Johnson Vaccine
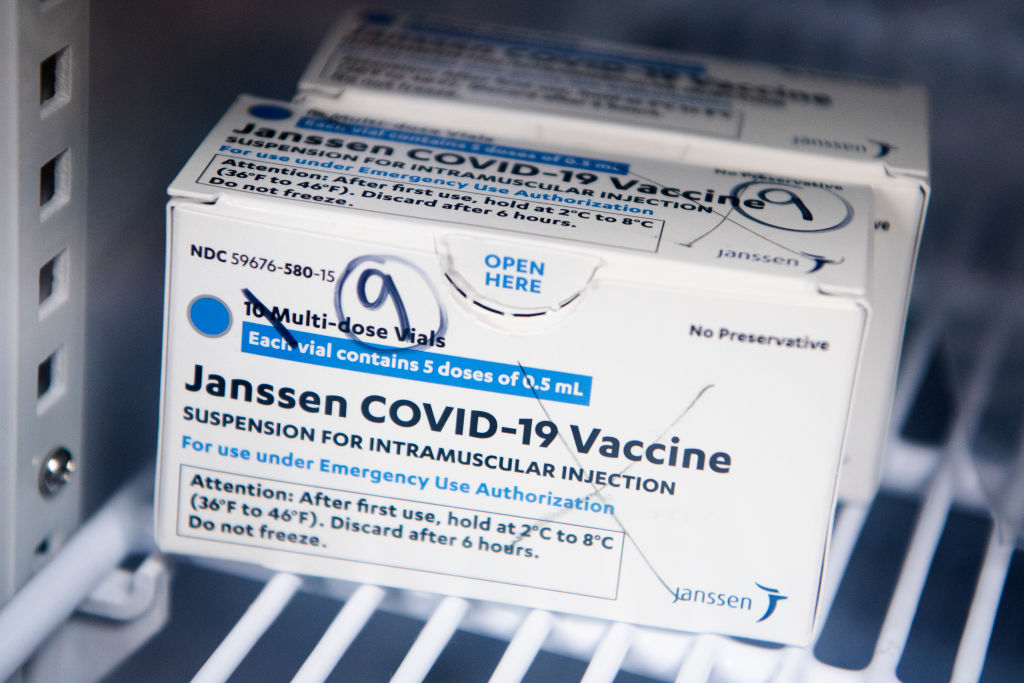
Source: Tom Williams / Getty
The FDA has announced the distribution of the Johnson & Johnson COVID-19 vaccine will be paused after six women in the U.S. had rare blog-clotting after receiving the vaccine. The women ranged in ages from 18 to 48, and while six people may not seem like a lot precautions are being taken swiftly. There have been approximately 6.8 million shots given with the majority of people not having issues.
The pausing of the Johnson & Johnson vaccine does not affect the other two vaccines from Pfizer and Moderna. But if you did receive the Johnson & Johnson shot and are having to severe stomach pain, leg cramps, or severe headaches you should call your healthcare provider right away. If you do not know what shot you received, check your CDC vaccination card where it is noted what brand shot you received. If you have an appointment scheduled to receive the Johnson & Johnson COVID-19 vaccine, it is advised to call your provider for further directions.
Get Breaking News & Exclusive Contest in Your Inbox:
[ione_media_gallery id=”3917798″ src=”https://newsone.com/” overlay=”true”]
The Latest:
- Hennessy Hook-Up: Win Tickets To See Lizzo at Rodeo Houston!
- Man With Tourette's Sydrome Shouted N-Word, Vulgarities At 2026 BAFTA Awards
- Byron Donalds' Ja-Fakin Accent Revealed By Ex-Wife
- Texas 2026 Primary Election Guide
- Life Of The Mardi! A Gallery Of Beaded Baddies, Pretty Sheauxstoppers & Jazzy Belles Who Let The Good Times Roll At Mardi Gras 2026
- Reality Check Realness: Miss J Reflects On Recovery Woes & ANTM Regret
- Floyd Mayweather Jr. To Unretire After Mike Tyson Fight, Social Media Swears He’s Broke
- 'Right Man, Wrong Time': Kayla Nicole Speaks On Travis Kelce Relationship Ending
- J. Press Brought Out The Zaddies To The Runway At New York Fashion Week
- Teddy Riley Dragged For Call To Forgive R. Kelly, Apologizes For Planning New Music With Convicted Crooner
CDC & FDA Recommend Pausing Johnson & Johnson Vaccine was originally published on mycolumbusmagic.com













